The collection, curation, and management of Regulatory Intelligence (RI) is key to the effective functioning of Life Sciences companies at various levels: understanding policies and their implications for the development of business strategies, understanding the regulatory landscape to define regulatory strategies, and understanding submission requirements to make effective product registrations and submissions in various markets and seeking approvals.
Regulatory Value Chain
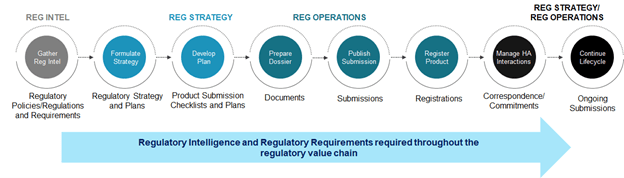
The end-to-end process of managing drug submissions and approvals, referred to as the regulatory value chain, is shown below. Regulatory intelligence and requirements are key to the effective functioning of this value chain throughout the lifecycle of the product registration process. Lack of complete and timely understanding of the requirements may cause significant rework and filing delays, potential rejection by health authorities, and loss of revenues.
Current Practices are Ineffective
Understanding regulatory policies and requirements drives strategy and planning activities while understanding submission requirements drives the effective creation of documents, submissions, and product registrations with health authorities.
Ultimately, the approval and lifecycle management of products depends on a continuous understanding of regulatory and submission requirements as they evolve and change in the market. So, keeping abreast of fast-changing regulations and their impact is key to successful product launches.
Managing regulatory intelligence is one of the key components of RIM. We strongly believe that current solutions in the form of commercially available regulatory intelligence subscription databases filled with PDF documents, homegrown SharePoint sites and databases are not sustainable in the long run with the potential for significant re-work, delayed or missed filings and lost revenues.
Transforming Regulatory Information Management with ReALM®
With several decades of experience working to address regulatory information management challenges in the Life Sciences industry, Orion has developed a SaaS platform called ReALM® (Regulatory Affairs Lifecycle Management). ReALM® supports four RIM modules with the underlying premise that RI is key to the effective functioning of various regulatory functions within the value chain.
- ReALM® Regulatory Intelligence module supports the collection, curation, and management of regulatory intelligence and requirements such as regulations, health authority guidelines, subject matter expert interpretations of guidelines, prior company knowledge and best practices, etc., providing a rich environment for better regulatory strategy and planning across different markets.
- ReALM® Submission Planning supports the creation of detailed submission plans at the market level and at each regulatory activity or filing level, supported by regulatory intelligence and requirements, and also enables the detailed planning of tasks and assignments to various individuals for completion.
- ReALM® Registrations supports the planning and tracking of product registrations along with lifecycle management activities, again driven by access to regulatory requirements for each market. In addition,
- ReALM® Interactions supports the management of health authority interactions such as correspondence, commitments. and company responses to health authorities and is available along with ReALM® Registrations module.
Business Impact Examples
Using a SaaS platform like ReALM® can transform the regulatory value chain. Here are a few examples of the impact to life sciences companies.
A fast-growing contract research organization (CRO) in the Asia-Pacific region supports regulatory filings on behalf of global pharmaceutical companies with no local presence in certain countries there. The CRO was challenged with managing information about product filings, health authority interactions, product registrations and lifecycle management activities, all contained in spreadsheets involving 13 markets. We configured and deployed the ReALM® platform to manage product and site registrations and health authority correspondence. More than 800 products and thousands of variations are managed within this SaaS solution. The CRO has successfully increased visibility into the entire product registration portfolio by enabling 13 markets to have access to a single RIM environment, increasing efficiencies and compliance, and reducing errors.
A large global pharmaceutical company was challenged with managing global regulatory requirements and intelligence for clinical trials and regulatory submissions. We configured and piloted a ReALM® platform to manage actionable regulatory intelligence for clinical trials and regulatory submissions. We also showcased capabilities to produce actionable regulatory intelligence for CTAs, NDAs, life cycle management submissions, CPPs, etc. ReALM® provided up-to-date regulatory intelligence for various regulatory activities managed in a single repository with the ability to create submissions checklists for specific products, which can then be transformed to a table of content plans for implementation.
What This Means for Your Business
It is no longer necessary to manage an important function like RIM with regulatory intelligence gathered in spreadsheets and pdfs. With Orion’s ReALM® platform, life science companies can gain actionable regulatory intelligence driving regulatory activities across the value chain and realize a significant improvement in efficiencies, greater regulatory compliance, and reduced filing delays.
Learn more about our cloud-based technology solution, ReALM®.
Contact us to find out how you can implement ReALM® into your RIM function.